PROJECT SUMMARY
We are Salut.
Salut Products has been established to commercialise the work of Dr Trudi Collet and her team, the Innovative Medicines Group, located at the Queensland University of Technology. The Innovative Medicines Group has been working on several novel compounds for mosquito-borne viruses. The Innovative Medicines Group has been working on several novel compounds for mosquito-borne viruses.
Salut believes that these compounds may also have Coronavirus therapeutic properties.


OUR FOCUS
Targeting Mosquito-borne viruses and Coronaviruses
Salut’s focus are on novel compounds for mosquito-borne viruses and coronaviruses targeting:
- Dengue, Zika and West Nile Virus
- Coronavirus (COVID-19)
Salut’s mandate is to significantly change the treatment and prophylaxis of viral diseases. This includes Dengue fever (and other Flaviviridae diseases) and Coronaviruses with a small molecule oral treatment for these debilitating conditions.
DENGUE VIRUS
what is the impact?
An emerging threat
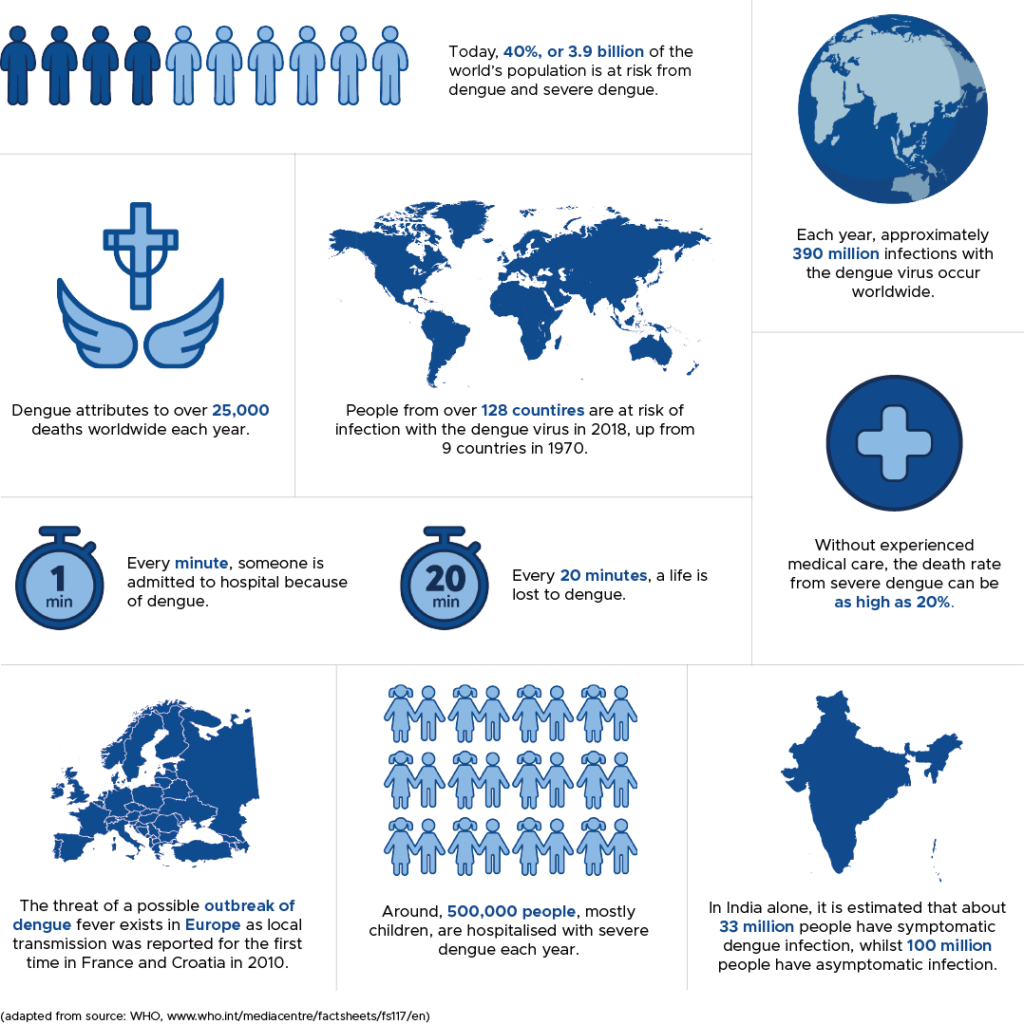
Three of the mosquito-borne viruses Dengue (DENV), Zika (ZIKV) and West Nile (WNV) contribute to approximately 400 million new infections every year, with 40% of the world’s population at risk.
Scientific modelling indicates that by 2080, 6 billion people will be at risk of Dengue infection with substantial increases in incidence across Africa, south-eastern USA, coastal Japan, China and Australia.
Although largely asymptomatic, arbovirus infections have significant societal and economic impacts globally. Attempts to treat DENV infection take the form of vaccines (prevention) or small molecule inhibitors (treatment).
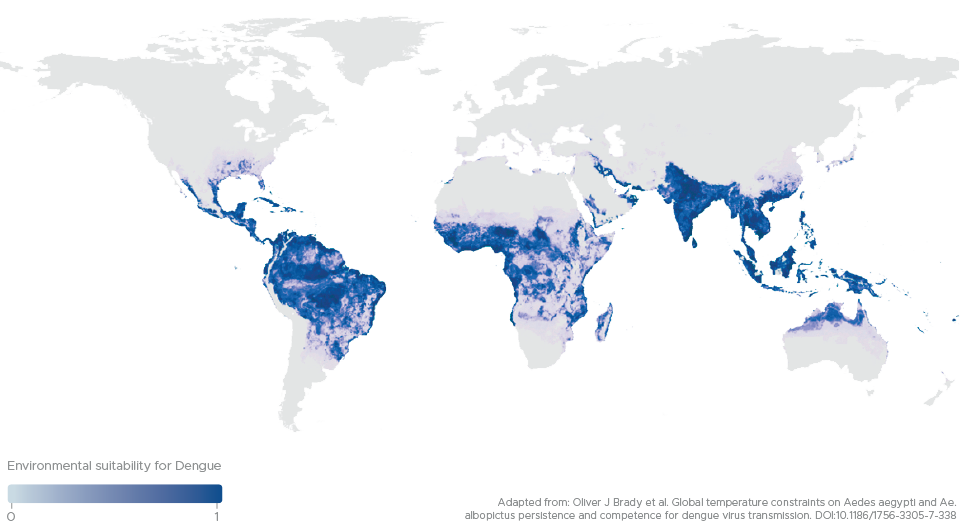
Dengue around the world
OUR SOLUTION
Novel first-in-class small molecule antivirals for Dengue and Coronaviruses.
The Salut Group has a mandate is to significantly change the treatment and prophylaxis of viral diseases with a simple small molecule oral treatment.
Research data to date has been very positive: our programs have demonstrated significant clinical efficacy and utility in in-vitro, cellular and meg analysis.
- Novel first in class small molecule antiviral for Dengue, targeting NS3 RNA helicase and NS5 RNA-dependent RNA polymerase.
- Recent docking studies and analysis have been performed with COVID-19 and it looks to be able to bind to and inhibit the main protease and therefore inhibit viral replication
- Initial selected candidates show good affinity across all four sub-types of Dengue
- Antiviral therapy results have been excellent in immuno-focus assays in Dengue, Zika and Kunjin (the Australian variant of the West Nile virus).
- Wide potential due to these targets being highly conserved across the flaviviridae family of viruses.
- Highly effective with excellent therapeutic and prophylactic potential.
- Simple, well characterised small molecule rather than vaccine, which to date, have been problematical.
Salut is now resolved to finalise preclinical animal trials on the Dengue fever molecule; and continue research into other indications and intellectual property at a high level until further tailored resources are available.
Following recent analysis, Salut is about to start an in-vitro program for coronavirus COVID-19 at QIMR (Queensland Institute of Medical Research) with a rapid move into animal models if this is successful.

OUR PROGRESS
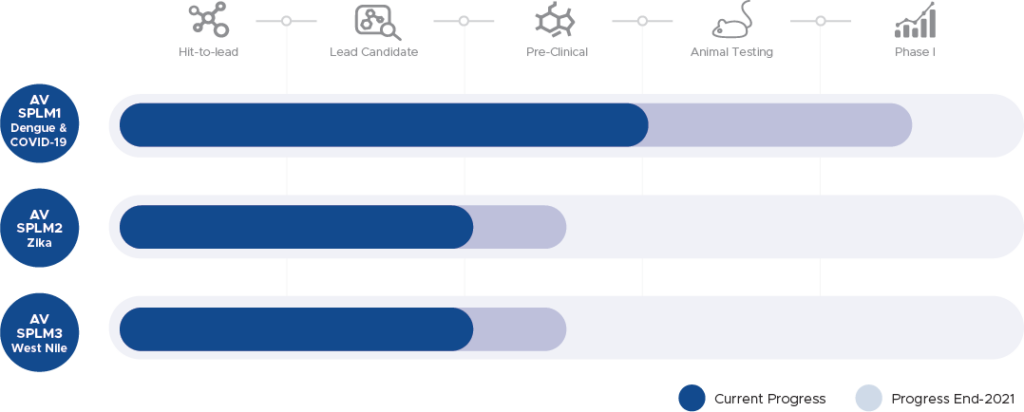
Product Pipeline
Salut will concentrate over the next two years on developing its current pipeline products:
- SPLM1 (Lead Candidate) – Dengue & COVID-19
- SPLM2 – Zika
- SPLM3 – West Nile
The Company has the primary objective, by the end of Q1 2021 to be ready to initiate an Australian-based Phase I clinical trial in DENV with its lead candidate SPLM1.
Salut’s Mid-Term objective to form a business relationship with a major pharmaceutical or biotechnology company by the end of 2021. Salut will be working on securing a licensing transaction and potentially be listed as a public company to accelerate commercialisation.
THE TEAM
Our Board
Dr. Stewart Washer
Chairman
Stewart has CEO and Board experience in medical biotech and cannabinoid companies. He is currently the Executive Chairman of Emerald Clinics, Chairman of Botanix (ASX:BOT), Director of Zelda Therapeutics (ASX:ZLD) and Chairman of Orthocell (ASX:OCC). Stewart is also Founding Chairman and current Director of Cynata Therapeutics (ASX:CYP), stem cell therapies.Mr. Bob Atwill
Managing Director
Bob is a Principal of Eaton Square and has over 30-years’ experience in the global pharmaceutical, biotechnology, cellular therapies, IT, medical device, diagnostic, healthcare services, life science and consultancy sectors. He has been a CEO for over 17 years and a Senior Executive and Corporate Officer in NYSE, LSE and ASX organisations since 1997.Mr. Brendan Butcher
Director
Brendan has 16 years translational research experience, from lead Scientific Technical roles at Q-Gen through to General Management and as CEO role within the Health Focus Group. Brendan is responsible for the Biodiscovery programme that has identified the SPLM compound.Dr. Stuart Boyer
Director
Stuart is Practice Group Director for the Life Sciences and Chemistry Group at Griffith Hack. Stuart’s background includes virology and molecular immunology. As a patent attorney he has worked with many ASX and non-listed biotechnology companies in developing their IP in areas from cellular therapies to small molecules. Stuart also consults on IP strategies, which support commercial goals.Dr. Mark Baldock
Director
Dr Mark Baldock is a Medical Doctor and a Registered Pharmacist. He has been buying, selling and establishing pharmacy businesses in Australia for the past 26 years and is extremely experienced and knowledgeable regarding the Australian pharmacy industry.THE TEAM
Management & Collaborators
Bob Atwill
Managing Director / CEO
Bob is a Principal of Eaton Square and has over 30-years experience in the global pharmaceutical, biotechnology, cellular therapies, IT, medical device, diagnostic, healthcare services, life science and consultancy sectors. He has been a CEO for over 17 years and a Senior Executive and Corporate Officer in NYSE, LSE and ASX organisations since 1997.Brendan Butcher
Director
Brendan has 16 years translational research experience, from lead Scientific Technical roles at Q-Gen through to General Management and a short-term CEO role with Health Focus Group. Brendan is responsible for the Biodiscovery programme that has identified the SPLM compound.Dr. Mike West
COO
Mike has a wealth of experience in global pharmaceutical and biotechnology industries. He has worked for US, EU and ANZ Biotech companies. He has a PhD in Chemistry and has taken a number of pharmaceutical drugs from bench to market. In doing so he has been involved in the pre-clinical development, scaleup, manufacturing and drug product development, as well as several clinical trials. He is also an Associate Professor within a major Australian University.Darren Scotti
CFO and Company Secretary
Darren is a CPA with over 24 years’ experience specialising in business expansion, financial modelling and negotiating Darren has honed his skills in a wide range of industries and diverse businesses from large multi-nationals to small to medium owner-operator concerns. He is currently the CFO of Enlitic and prior to that was the initial CFO of Nohla Therapeutics.Dr. Trudi Collet
Collaborator, QUT R&D
Trudi, a biochemist, was awarded her PhD from QUT in 2007. Currently, Trudi is leader of the Innovative Medicines Group (IMG), which is based at the Kelvin Grove campus of QUT. The IMG specialises in the pharmaceutical potential of Australian native plants and derived compounds for their use as novel therapeutics for global infections and diseases.